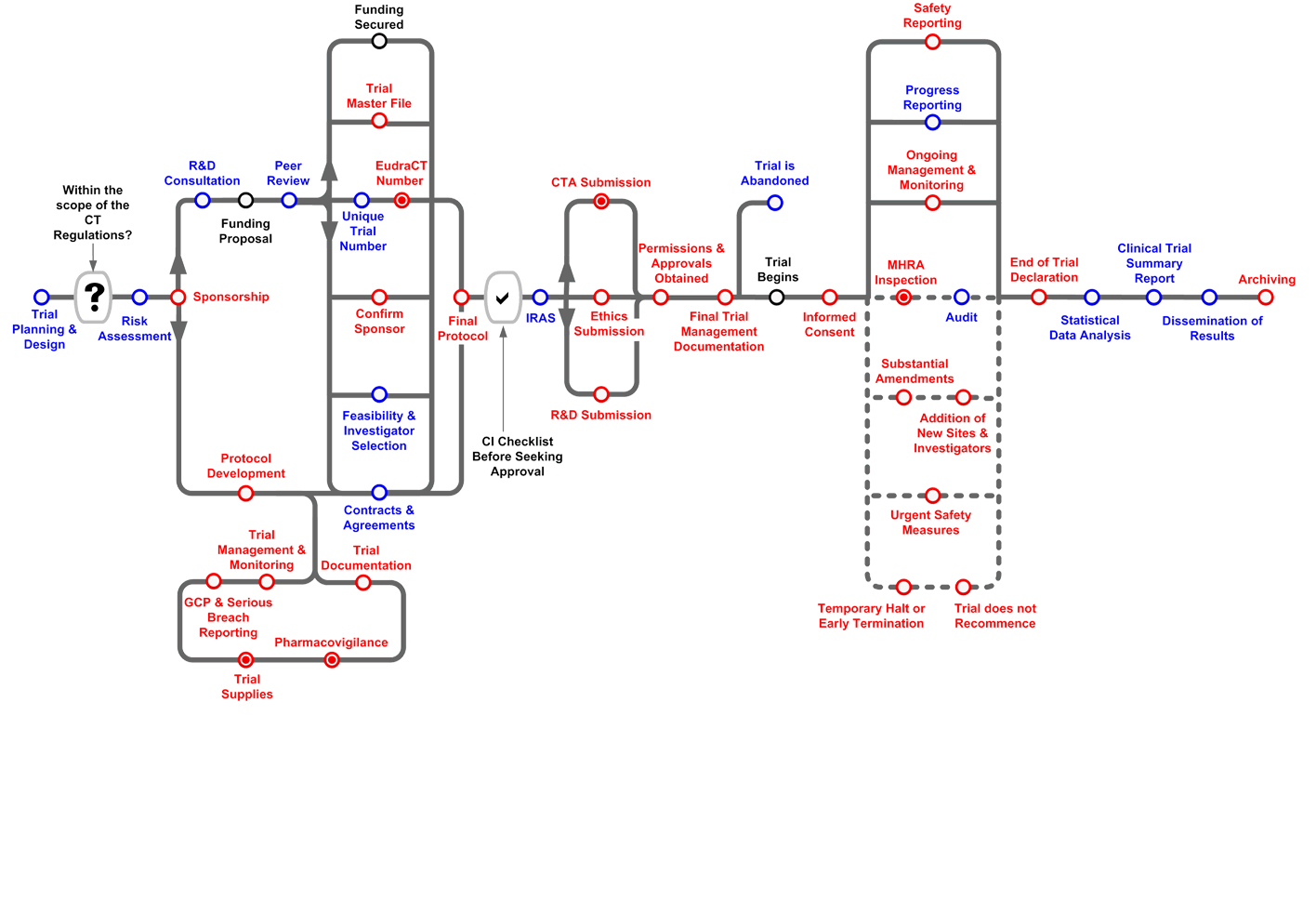
Routemap
The Clinical Trials Toolkit is an interactive colour-coded routemap* to help navigate through the legal and good practice arrangements surrounding setting up and managing a Clinical Trial of an Investigational Medicinal Product (CTIMP). The routemap distinguishes between legal and good practice requirements, and indicates which aspects of these are relevant to wider clinical research in general. It includes an overview of trial practices, along with more detailed information available at ‘stations’ along the route.
*Mobile users will be able to access the stations from the list shown
Download a copy of the Clinical Trials Toolkit routemap (pdf, 221.33 KB).
Skip the Routemap image and continue reading
 Trial Planning & Design
Trial Planning & DesignWithin the scope of the Clinical Trial Regulations?
Within the scope of the Clinical Trial Regulations?Risk Assessment
Risk AssessmentSponsorship
SponsorshipProtocol Development
Protocol DevelopmentGCP & Serious Breach Reporting
GCP & Serious Breach ReportingTrial Management & Monitoring
Trial Management & MonitoringTrial Documentation
Trial DocumentationTrial Supplies
Trial SuppliesPharmacovigilance
http://www.nets.nihr.ac.uk/funding/
PharmacovigilanceR&D Consultation
R&D ConsultationFunding Proposal
Funding Proposal Peer Review
Peer ReviewFunding Secured
Funding SecuredTrial Master File
Trial Master FileUnique Trial Number
Unique Trial NumberEudraCT Number
EudraCT NumberConfirm Sponsor
Confirm SponsorFeasibility & Investigator Selection
Feasibility & Investigator SelectionContracts & Agreements
Contracts & AgreementsFinal Protocol
Final ProtocolCI Checklist Before Seeking Approval
CI Checklist Before Seeking ApprovalIRAS
IRASCTA Submission
CTA SubmissionEthics Submission
Ethics SubmissionTrial begins
R&D SubmissionPermissions & Approvals Obtained
Permissions & Approvals ObtainedFinal Trial Management Documentation
Final Trial Management DocumentationTrial is Abandoned
Trial is AbandonedTrial Begins
Trial BeginsInformed Consent
Informed ConsentSafety Reporting
Safety ReportingProgress Reporting
Progress ReportingOngoing Management & Monitoring
Ongoing Management & MonitoringMHRA Inspection
MHRA InspectionAudit
AuditAddition of New Sites & Investigators
Addition of New Sites & InvestigatorsSubstantial Amendments
Substantial AmendmentsUrgent Safety Measures
Urgent Safety MeasuresTemporary Halt or Early Termination
Temporary Halt or Early TerminationTrial does not Recommence
Trial does not RecommenceEnd of Trial Declaration
End of Trial DeclarationStatistical Data Analysis
Statistical Data AnalysisClinical Trial Summary Report
Clinical Trial Summary ReportDissemination of Results
Dissemination of ResultsArchiving
Archiving
Trial Planning & Design
Trial Planning & DesignWithin the scope of the Clinical Trial Regulations?
Within the scope of the Clinical Trial Regulations?Risk Assessment
Risk AssessmentSponsorship
SponsorshipProtocol Development
Protocol DevelopmentGCP & Serious Breach Reporting
GCP & Serious Breach ReportingTrial Management & Monitoring
Trial Management & MonitoringTrial Documentation
Trial DocumentationTrial Supplies
Trial SuppliesPharmacovigilance
http://www.nets.nihr.ac.uk/funding/
PharmacovigilanceR&D Consultation
R&D ConsultationFunding Proposal
Funding Proposal Peer Review
Peer ReviewFunding Secured
Funding SecuredTrial Master File
Trial Master FileUnique Trial Number
Unique Trial NumberEudraCT Number
EudraCT NumberConfirm Sponsor
Confirm SponsorFeasibility & Investigator Selection
Feasibility & Investigator SelectionContracts & Agreements
Contracts & AgreementsFinal Protocol
Final ProtocolCI Checklist Before Seeking Approval
CI Checklist Before Seeking ApprovalIRAS
IRASCTA Submission
CTA SubmissionEthics Submission
Ethics SubmissionTrial begins
R&D SubmissionPermissions & Approvals Obtained
Permissions & Approvals ObtainedFinal Trial Management Documentation
Final Trial Management DocumentationTrial is Abandoned
Trial is AbandonedTrial Begins
Trial BeginsInformed Consent
Informed ConsentSafety Reporting
Safety ReportingProgress Reporting
Progress ReportingOngoing Management & Monitoring
Ongoing Management & MonitoringMHRA Inspection
MHRA InspectionAudit
AuditAddition of New Sites & Investigators
Addition of New Sites & InvestigatorsSubstantial Amendments
Substantial AmendmentsUrgent Safety Measures
Urgent Safety MeasuresTemporary Halt or Early Termination
Temporary Halt or Early TerminationTrial does not Recommence
Trial does not RecommenceEnd of Trial Declaration
End of Trial DeclarationStatistical Data Analysis
Statistical Data AnalysisClinical Trial Summary Report
Clinical Trial Summary ReportDissemination of Results
Dissemination of ResultsArchiving
Archiving
Getting Started with the Routemap
The Clinical Trials Toolkit is designed to help understand the requirements of the UK Medicines for Human Use (Clinical Trials) Regulations, which together with its amendments, are referred to as the Clinical Trial Regulations within this Toolkit.
Users should note the following features;
- The positioning of the stations (from left to right on the routemap or from top to bottom on the station list) gives an indication of the critical path and process flow.
- Activities that may be undertaken in parallel are indicated on the routemap and explained within each station.
- By clicking on a station, you will be taken to a description of the process and a list of relevant resources and/or related stations.
- Station symbols can either apply to trials covered by the CT Regulations, or apply to all trials (please see the key to symbols).
- Where a station includes statutory requirements for CTIMPs, (even if the requirement is not a general legal requirement for non-CTIMPs) the station, by default, will be identified as a legal requirement.
- In many cases, good practice requirements may be considered mandatory even if the requirement does not have statutory force (e.g. by Funders or the NHS).
- Stations are grouped into four categories: Planning, Approval, Recruitment, and Close Out.
Before using the routemap, take time to think about your trial and its setting. Consider the following:
- The research question
The nature of the intervention will determine whether the trial is subject to the regulations. The routemap contains advice on management arrangements for different kinds of trial. - Where you are in the trial process
The key stages of the trial process are identified as ‘stations’. By selecting a station you will find one or more ‘resources’. The resources provide advice and links to other useful websites and resources. - The expertise of your trials team and organisation
Some resources within the Toolkit address the needs of trialists, whilst others are of particular interest to those involved with R&D management. Using the resources may reveal gaps in the capacity of your trials team or organisation to support trials to the required standards. Organisations vary considerably in how they approach their responsibilities to support high quality clinical trials. - The importance of Patient and Public Involvement
Consider how best to involve members of the public in your work. See INVOLVE’s briefing notes for researchers.
PPI guidance for Chief Investigators of UK surgical trials has been developed from the 'Patient and public involvement Intervention to enhance Recruitment and Retention in Surgical Trials' (PIRRIST) project.
Trials that began before 1 May 2004
The Medicines for Human Use (Clinical Trials) Regulations came into force on 1 May 2004. Any trials initiated before this date (i.e. trials which had a Clinical Trial Certificate (CTC) or Clinical Trial Exemption (CTX) or which had been notified under the Doctors and Dentists Exemption (DDX) scheme) and that were ongoing after 1 May 2004 were required to ensure that systems were put in place to comply with these regulations.
The MRC/DH Joint Project produced a Checklist for Ongoing Trials in 2004 which helped sponsors identify any immediate actions to be taken in relation to their trials. The MHRA also published advice on amendments or end of trial declarations for clinical trials that were originally CTX/DDX/CTC applications.

